Environment & Energy
Related: About this forumHydrogen Is Working and It’s Much Cheaper Than We Thought
TheEnergyCollective.com August 17, 2016 by David Thorpe
The technology that produces hydrogen using renewable electricity has already passed crucial regulatory tests for grid balancing in a commercial environment, despite what I said here a month ago.

For over 30 years the prophets of green energy have been promoting the idea that the “hydrogen age” is just around the corner. The gas is abundant in the form of water, molecules of which possess two hydrogen atoms for every oxygen atom.
Making it from water using electrolysis releases only oxygen and no pollutants. It can then be burnt in any suitable boiler, cooker or vehicle and used in fuel cells. All we have to do is get it to the right place at the right time at the right price.
The problem has always been the right price, which provides the market incentive for investment in the necessary infrastructure...snip
Power-to-gas
The process is called power-to-gas (P2G) and it is useful when too much renewable electricity is being produced compared to the demand that exists at that moment. Instead of it going to waste it could be used to produce hydrogen as a form of energy storage and used when required...snip
Advantages of hydrogen over batteries...snip
Read More: http://www.theenergycollective.com/david-k-thorpe/2385923/hydrogen-is-working-and-its-much-cheaper-than-we-thought
http://www.theenergycollective.com/
Did You Know: There are 22 Litres of Hydrogen in ONE TABLESPOON of water

TexasTowelie
(111,847 posts)At 9.5 BTU/liter, that's 209 BTU's.
For comparison, 22 liters of gasoline holds 663,000 BTU's.
Using volume with a gas of very low energy density isn't exactly the best unit to measure it's potential by.
http://www.democraticunderground.com/112773946 (post #7)
nationalize the fed
(2,169 posts)Do you have any idea how proud I am to be a Hydrogen advocate while the majority here have no idea what is going on with the tech?
These threads will be fun to look at a few years from now. I guarantee it.
Meanwhile, what has the US done for energy independence lately?
NOT A GOD DAMN THING
The Democratic Nominee has just chosen Salazar to head the "transition" team. ROFL
CentralMass
(15,265 posts)energy in electric vehicles then using electricity to create hydrogen via electrolysis of water. After the hydrogen is produced it must be compressed in storage tanks requiring more electrical energy.
Battery powered.electric vehicles are 3 to 6 time more efficent at using electrical power in this usage.
Hydrigen is not a fuel it is an energy carrier. It takes energy to create it. Either via electrolysis of water or by reforming fossil fuels like natural gas to convert it to hydrogen and carbon by products
Niether process is efficient or provide a cost advantage over electric vehicle or efficient fossil fuels powered vehicle such as hybrids.
All of these experimental hydrogen fueling stations in states like California are subsidized. The cost of the hydrogen is not the real cost. It is being sold at a loss or given away in these pilot programs.
.
nationalize the fed
(2,169 posts)If it was, no one would buy any car that gets less than the current max MPG, would they.
But people buy cars every day that get less mileage than the max. Because they trade efficiency for something else.
If someone's time is worth $300 or $400 dollars per hour do you think they would like to refuel their electric car in 3-5 minutes or wait around at a supercharger for 45 minutes?
Hydrogen makes it possible for those whose time is valuable to drive electric without the constant time drain of recharging. Also those who can't put a fast charger in their garage.
What form of energy does not take energy to obtain? Look at how much energy it takes to frack for natural gas. A crew, each of which makes probably $50,000/yr or more, gas for vehicles, energy to drill, energy to pump water in the earth and so forth. All it takes to make hydrogen is water and electricity. And if the electricity comes from wind or solar it's free, after the equipment is paid for.
They aren't "experimental" any more. You can drive throughout Denmark on hydrogen made from renewable energy. Germany is right now in the process of installing 400 hydrogen stations. http://h2me.eu/2016/05/05/germany-h2-mobility-targets-400-hydrogen-fueling-stations-by-2023/
$2.00 or less per Kg of H2- that's what is coming. And it will change the world.
Battery only EV's are dinosaurs. Hydrogen is The Next Big Thing.
https://www.youtube.com/user/H2FCHannover/videos
TreasonousBastard
(43,049 posts)Half way right.
You can't compare gaseous hydrogen with a liquid fuel by volume. It has to be by weight.
Here's a hydogen advocate's takeo nit:
http://www.solarhighway.org/HydrogenFacts.html
h2-- 60,000 btu per pound
gasoline 18,000 btu per pound
And here's a more neutral chart that claims the same:
http://cta.ornl.gov/bedb/appendix_a/Lower_and_Higher_Heating_Values_of_Gas_Liquid_and_Solid_Fuels.pdf
Now, will a real chemist or physicist kindly explain why such a flimsy thing as hydrogen has such a high BTU level? Do all those carbon atoms in hydrocarbons mean little?
And, yes, I'm aware of the trade-off in the energy used to compress the hydrogen-- the point made by the first guy is that you can reduce compression costs.
OKIsItJustMe
(19,937 posts)Last edited Thu Aug 18, 2016, 09:52 AM - Edit history (2)
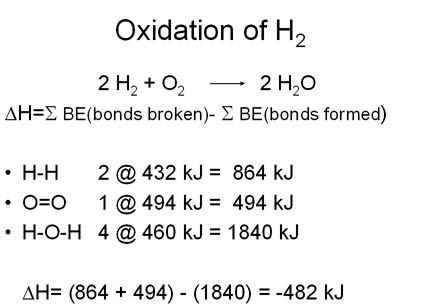
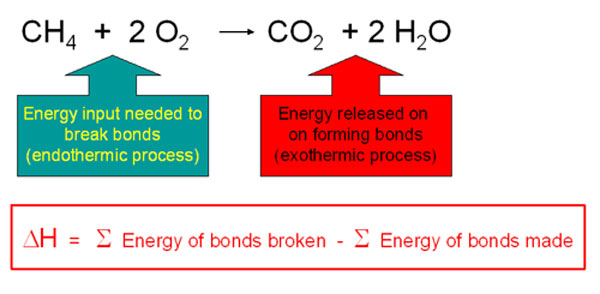
Breaking bonds takes energy, making bonds releases energy. If the bonds you make release more energy than the bonds you break, the overall reaction produces energy.
Here’s a comparison of hydrogen and methane. Starting with two hydrogen molecules and one oxygen molecule, producing two molecules of water, two H-H bonds and one O=O bond are broken and then four O-H bonds are created:
https://www.wou.edu/las/physci/GS361/Energy_From_Fossil_Fuels.htm
…
…
…
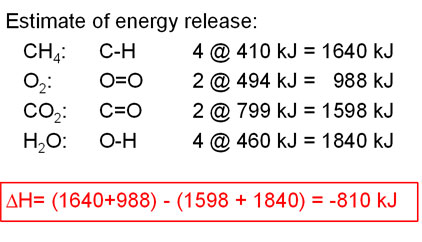
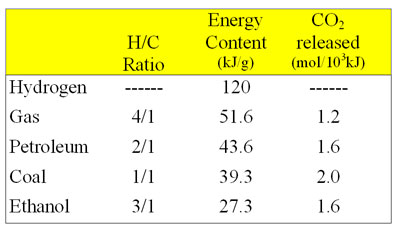
Why the big difference in kJ/g? Keep in mind that carbon (¹²C) is a good deal more massive than hydrogen (¹H).
TexasTowelie
(111,847 posts)The correct unit for comparison is the mole because they would contain the same number of molecules. When comparing the btu per pound you actually have far more molecules of hydrogen than you do of gasoline (octane) because the molecular weight of diatomic hydrogan is 2.0 grams/mole while the molecular weight for octane is 114 grams/mole.
TreasonousBastard
(43,049 posts)you want to bring moles into it? I haven't taken a chemistry course since High School.
Here's the thing-- if I got a tank of liquid hydrogen and a tank of gasoline, which will do more work? Since it's a lot of energy to liquify hydrogen, what density of the gas is the most efficient pressure to get the most work done? And how does that compare with, say, methane?
I have no argument with hydrogen, and have even played around with it a little (electrolysis is easy), but any discussion of it has to end up with practical uses and comparisons.
OKIsItJustMe
(19,937 posts)One mole of methane (at “standard temperature and pressure”) has roughly the same volume (22.4 liters) as one mole of hydrogen.
http://www.science.uwaterloo.ca/~cchieh/cact/c120/idealgas.html
On the other hand, one mole of methane weighs sixteen times as much as one mole of hydrogen.
http://www.convertunits.com/molarmass/Methane : Molar mass of CH4 = 16.04246 g/mol
http://www.convertunits.com/molarmass/Hydrogen : Molar mass of H = 1.00794 g/mol
OKIsItJustMe
(19,937 posts)Last edited Thu Aug 18, 2016, 12:04 PM - Edit history (1)
The Saturn V used kerosene “RP-1” in its first stage, and (liquid) hydrogen “LH₂” in its upper stages.
Why? Well, different priorities:
http://history.nasa.gov/SP-4206/ch6.htm
The upper stages of such a vehicle were critical to the eventual success of the mission-especially the top stage, which inserted the payload into the final, stabilized orbit. Douglas engineers were emphatic. "The overall performance of the end-stage has greater influence than the primary stages. The Saturn V launch vehicle for the lunar mission requires 50 pounds (23 kilograms) of booster weight at liftoff for each pound of payload injected into a translunar trajectory," they explained. "Without high-energy upper stages this factor would be significantly greater." The key to these high-energy stages was liquid hydrogen as the fuel. An engineer from Douglas, the eventual contractor of the S-IV and the S-IVB, summed up the significance of the decision to use liquid hydrogen. "The combination of hydrogen and oxygen for propellants made the moon shot feasible," he declared. "Its use in upper stages results in a significant increase in performance over the propellant combinations of oxygen and kerosene then in use in first-stage boosters."
…
http://history.nasa.gov/SP-4206/ch7.htm
When the contract to build the biggest stage of the Saturn V, the S-IC first stage, was awarded to Boeing on 15 December 1961, general outlines of the first-stage booster were already fairly well delineated. The main configuration of the S-IC had already been established by MSFC, including the decision to use RP-1, as opposed to the LH₂ fuel used in the upper stages. Although LH₂ promised greater power, some quick figuring indicated that it would not work for the first stage booster.
Liquid hydrogen was only one half as dense as kerosene. This density ratio indicated that, for the necessary propellant, an LH₂ tank design would require a far larger tank volume than required for RP-1. The size would create unacceptable penalties in tank weight and aerodynamic design. So, RP-1 became the fuel. …
For the upper stages, the higher energy/mass ratio of hydrogen made it the winner. For the first stage, the higher energy/volume ratio of RP-1 made it the better choice.
The fundamental laws of physics remain the same today.
OKIsItJustMe
(19,937 posts)Well, a UK tablespoon comes close… (assuming the hydrogen is brought to “standard temperature and pressure.”)
However, the question is, “So what?” To produce that mole of hydrogen requires a great deal of energy; somewhat more than you will get back if you recombine it with 11 liters of oxygen to produce the original mole of water.
I believe hydrogen has great potential as a way to store energy, but I don’t see where this particular bit of trivia is meaningful.