Environment & Energy
Related: About this forumTrump's favorite techie thinks there should be 'more open debate' on global warming
https://www.yahoo.com/news/facebook-board-member-says-must-173302778.htmlThis is all this according to Peter Thiel, a Facebook board member and influential advisor to President Donald Trump.
Speaking on Tuesday at a prominent energy industry conference in Houston, Thiel said he is skeptical of mainstream climate science that has shown the Earth is warming primarily due to human activities, such as the burning of fossil fuels for energy.
"I don't know whether I am an extreme skeptic on climate change, but I have my doubts about the extreme ways that people try to push it through," Thiel said, according to reporting from Ben Geman of Axios as well as a recording of the session.
Fuck off, Thiel. Just fuck off.
Botany
(70,524 posts)So CO2 has more mass then O2 so it can hold more energy aka heat.
Fuck these "keep an open mind" assholes.
https://www.nytimes.com/2016/09/04/science/flooding-of-coast-caused-by-global-warming-has-already-begun.html?_r=0
caraher
(6,278 posts)Water vapor has a molecular mass of 18 compared to 32 for O2, but O2 contributes nothing to trapping infrared radiation while water vapor is a much bigger absorber of infrared radiation than CO2 (mainly because there's more of it in the atmosphere).
The reason the dominant constituents of the atmosphere (N2, O2) don't trap infrared light is that homonuclear diatomic molecules can't make a single-photon transition that is purely vibrational. The visible and near-visible transitions are dominated by electronic energy levels while the transitions at infrared wavelengths are between vibrational levels. Those are forbidden in diatomic molecules with identical nuclei like most N2 and O2 molecules, so well over 90% of the atmosphere is made of molecules that do not absorb infrared. The greenhouse effect arises from the trace gases that, because of their structure, do absorb and emit infrared.
But... yes, fuck them ![]()
Botany
(70,524 posts)and are you trying to say that mass of a molecule has less to do with
the ability of a molecule to being able to hold onto energy aka heat introduced
into the molecule then the P-chem make up of the molecule?
caraher
(6,278 posts)Of course CO2 is more massive than O2 (by the mass of 1 carbon, of course!).
The warming problem isn't that we add molecules to the atmosphere that perhaps displace others that "hold" more thermal energy, or simply add to the number of molecules in the air. It's how the radiation balance of the atmosphere changes with the presence of more molecules that can absorb light at the wavelengths associated with 300 Kelvin background radiation.
The essence of the greenhouse effect is that an airless Earth has a steady-state energy balance that looks like this: the planet absorbs the solar blackbody spectrum, peaked at visible wavelength, and re-radiates into space its own blackbody spectrum, peaked in the infrared, such that the inflow and outflow of energy are equal. During warming, the balance is off; less energy radiates into space than Earth absorbs from the sun, because atmospheric gases absorb more of the IR, and when they re-emit the energy, some of it goes downward rather than into space. This warms both the atmosphere and the surface, which in the long run pushes the planet toward a new equilibrium where, thanks to higher temperatures, the surface emits more infrared.
This site seems to give a pretty thorough explanation and there's a Java applet that gives a nice interactive visual of the overall process.
Ultimately, the pchem matters not so much because the effect is primarily about a molecule's ability to hold onto energy, but the molecule's tendency to intercept and redirect the energy that would otherwise flow back out into space.
Botany
(70,524 posts)So does this model also explain why methane, CH4 with a m.w. of 16 vs
O2 with a m.w. of 32 as being a greenhouse gas?
BTW one model that scares the hell out of me is that the loss of polar ice caps
the solar energy that used to bounce back because of albedo effect now goes right
into the oceans.
The main thing is that molecular weight isn't really the issue except indirectly. What matters is the tendency to absorb (or not) infrared light, which can have a sort of very indirect correlation with molecular weight. But CH4 is a great illustration - per molecule it is a far more potent greenhouse gas than CO2, and it's all about the infrared absorption spectrum. That has more to do with how many ways there are for a molecule to bend, stretch and vibrate than with its actual mass. The indirect correlation is that molecules with more atoms both tend (on average) to weigh more and to have more ways to bend, stretch and vibrate. So things like CFCs are potent greenhouse gases not so much because they are heavy as because their structure allows for absorption at so many different infrared wavelengths.
Botany
(70,524 posts).... because chlorophyll* has lots of carbon atoms in its molocule and one of chlorophyll's
purposes is to capture photons of light energy.
* C55H72O5N4Mg
caraher
(6,278 posts)It's important to remember that the function of chlorophyll in absorbing visible light is very different from what's happening in absorbing IR radiation. Fundamentally yes, both involve the interaction of electromagnetic radiation with a molecule, which results in some commonalities. But the nature of the energy exchange is quite different, and molecules like chlorophyll have some fascinating electronic characteristics that contribute to their ability to transform light energy into biologically-useful chemical forms.
Botany
(70,524 posts).... inside a chloroplast by chlorophyll which is powered by photons of visible light is different
then absorption of infra red radiation into (or around ??) molecules that we call green house gases
but what I wonder does the basic structure of carbon lend itself to the capture of energy? Or is
the wavelengths of energy .... visible light vs IR radiation ..... so different that you can not
talk about their commonalities as per their interaction with carbon atoms found in molecular forms?
BTW is it possible to capture atmospheric CO2 and then break the molecule into organic carbon
and O2?
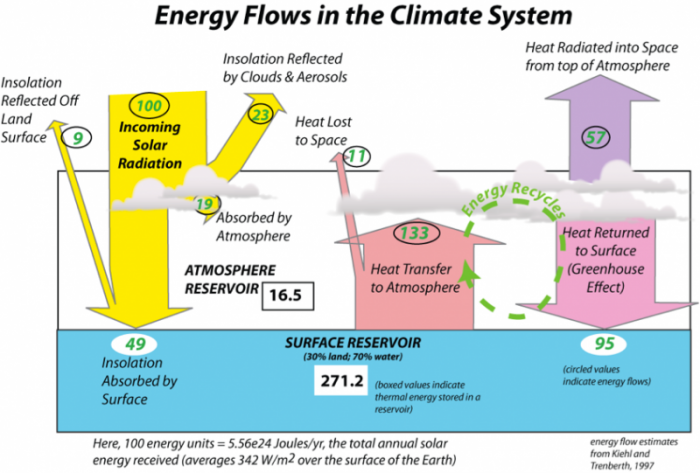
http://www.grida.no/publications/vg/climate/page/3057.aspx one of my favorite graphs.