Science
Related: About this forumPorous Rhodium Copper Nanospheres.
Last edited Wed Jan 24, 2018, 12:14 AM - Edit history (1)
In my electronic files, I have a copy of the Second Edition of Bradley Fahlman's wonderful text Materials Chemistry - a Third Edition either has, or is about to be released - which in its Appendix B reproduces a December 1959 lecture by Richard Feynman entitled "There's Room at the Bottom," in which he discusses a putative world in which it is possible to print the Encyclopedia Britannica on the head of a pin.
His speech began like this:
As soon as I mention this, people tell me about miniaturization, and how far it has progressed today. They tell me about electricmotors that are the size of the nail on your small finger. And there is a device on the market, they tell me, by which you can write the Lord’s Prayer on the head of a pin. But that’s nothing; that’s the most primitive, halting step in the direction I intend to discuss. It is a staggeringly small world that is below. In the year 2000, when they look back at this age, they will wonder why it was not until the year 1960 that anybody began seriously to move in this direction. Why cannot we write the entire 24 volumes of the Encyclopedia Brittanica on the head of a pin?
Let’s see what would be involved...
Later he continues in what seemed to him to be a perfectly reasonable possibility, although I'm not sure that everyone in his audience found it believable:
How much space would it take? It would take, of course, the area of about a million pinheads because, instead of there being just the 24 volumes of the Encyclopaedia, there are 24 million volumes. The million pinheads can be put in a square of a thousand pins on a side, or an area of about 3 square yards. That is to say, the silica replica with the paper-thin backing of plastic, with which we have made the copies, with all this information, is on an area of approximately the size of 35 pages of the Encyclopaedia. That is about half as many pages as there are in this magazine. All of the information which all of mankind has every recorded in books can be carried around in a pamphlet in your hand – and not written in code, but a simple reproduction of the original pictures, engravings, and everything else on a small scale without loss of resolution.
What would our librarian at Caltech say, as she runs all over from one building to another, if I tell her that, 10 years from now, all of the information that she is struggling to keep track of – 120,000 volumes, stacked from the floor to the ceiling, drawers full of cards, storage rooms full of the older books – can be kept on just one library card! When the University of Brazil, for example, finds that their library is burned, we can send them a copy of every book in our library by striking off a copy from the master plate in a few hours and mailing it in an envelope no bigger or heavier than any other ordinary air mail letter.
Now, the name of this talk is ‘There is Plenty of Room at the Bottom’ – not just ‘There is Room at the Bottom.’ What I have demonstrated is that there is room – that you can decrease the size of things in a practical way. I now want to show that there is plenty of room. I will not now discuss how we are going to do it, but only what is possible in principle – in other words, what is possible according to the laws of physics. I am not inventing anti-gravity, which is possible someday only if the laws are not what we think. I am telling you what could be done if the laws are what we think; we are not doing it simply because we haven’t yet gotten around to it.
1959...Remarkable...completely and totally remarkable.
As we all know the world that Feynman predicted in 1959 has come to pass. I can easily carry Fahlman's book, and a thousand books like it, plus thousands of copies of papers, photographs in my pocket on a thumb drive, which is something I do frequently.
What is more remarkable is that it is how possible to actually see things at an atomic scale. I had a nice tour with my son of the materials science department that he ultimately went to school, and the nice graduate student who conducted the tour took us to see a whole bunch of different microscopes, including an tunneling electron microscope where he displayed a photography of, um, atoms.
Whatever.
This is a brief note about a nanotechnical approach to utilizing vanishing resources more carefully.
I'm sure I've posted this periodic table in this space before, which shows the "critical elements" that are expected to run out, at least in traditionally processed ores accessible at low prices and utilized using current technology:
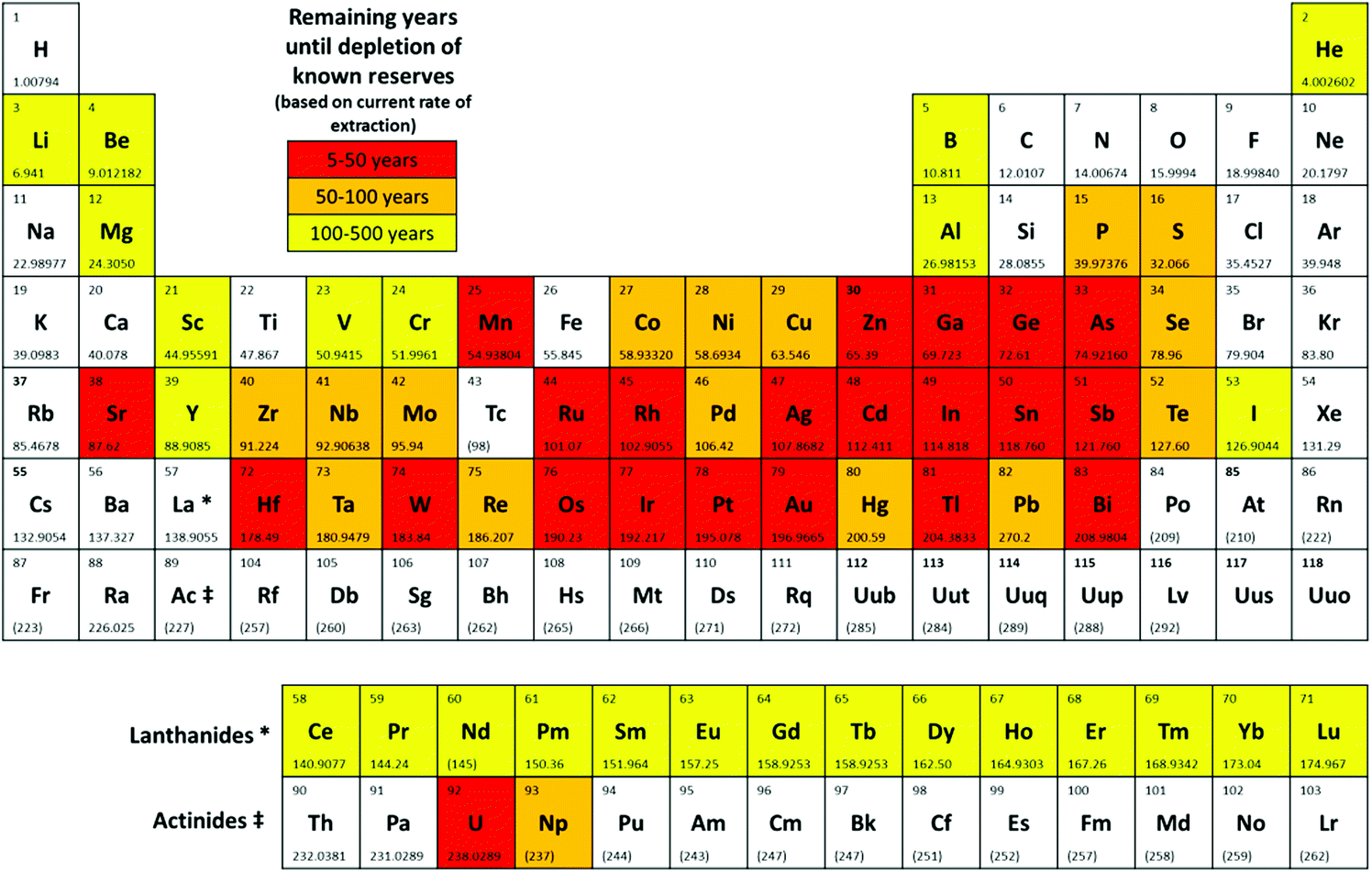
One may quibble a bit on the data this table represents - I have argued that because of its high energy density supplies of uranium are inexhaustible, for example - but I'm quite sure, the regrettable circumstance of "peak oil" not having come to pass - that in most cases the table explicates a serious threat to future generations; that many elements will become inaccessible.
One of the elements in red, element 45, rhodium is the one I'd like to discuss by pointing to a paper I came across today (and put on my thumb drive), this one: Mesoporous Bimetallic RhCu Alloy Nanospheres Using a Sophisticated Soft-Templating Strategy (Yamauchi et al, Chem. Mater., 2018, 30 (2), pp 428–435)
Rhodium is an important catalyst; it also serves as a minor constituent of alloys of profound technological importance.
According to another paper from a few years back (Electrochemical behavior of rhodium(III) in 1-butyl-3-methylimidazolium chloride ionic liquid, Srinivasan et al, Electrochimica Acta Volume 53, Issue 6, 15 February 2008, Pages 2794-2801) supplies of this element from terrestrial ores will actually be lower than the quantities available for isolation from used nuclear fuel.
The Yamuchi paper has a nice description of some of the important uses of rhodium catalysts:
The authors explore then, the Feynman solution, which is to make nanoparticles consisting of a copper rhodium allow in porous perforated spheres:
Scheme 1 as a graphic:

The caption:
A scanning electron microscope picture of the resulting balls:

The caption:
A tunneling electron microscope picture of them, touching on the atomic scale:

The caption:
Rhodium is a high melting metal; its alloy with copper melts lower. The authors suggest that this morphology actually overcomes the tendency for other copper rhodium systems to agglomerate, thus reducing the catalytic efficiency and lifetime.
Cool I think. This sort of thing should extend rhodium resources, at least until the human race comes to its senses and begins to utilize the valuable materials in used nuclear fuel.
Have a pleasant "hump day" tomorrow.
eppur_se_muova
(36,269 posts)NNadir
(33,525 posts)It made him think all about the Bravais lattices, a good thing for a kid going into materials science.
I mosey through it myself from time to time.
I'm kind of curious to take a look at the 3rd edition. I have both the 1st and 2nd in my files.
I remember running into the Feynman lecture; I was on a plane and clicking in my file around to make the time go by and boom!
Suddenly the plane was landing too soon.
It's an incredible read.