Polymers with controlled assembly and rigidity made with click-functional peptide bundles
The paper I'll discuss in this post is this one: Polymers with controlled assembly and rigidity made with click-functional peptide bundles, (Pochan et al, Nature 574, 658–662 (2019)).
I had a friend and colleague once who left his job because his company was telling him to make peptides on an industrial scale (ton quantities). He told me, offending me slightly, that he wanted to do "something other than dehydration reactions" that is remove water to make chemical bonds.
Those of us who are environmentalists complain, quite justifiably I think, about polymers, because single use plastics (and to a lesser extent multiple use plastics) are fouling the seas, land, and living systems at an increasing rate. Nevertheless in a very real sense, you are a polymer, or better put, a collection of polymers, since almost all of the molecules of which you are made are polymers.
There was a point in my career that I was a peptide chemist, and trust me, the chemistry of peptides (and their synthesis) is considerably more complex than simply removing water, with all due respect my friend's outstanding knowledge of our science. I will tell you that I spent a year of my life dealing with the huge difference between the behavior of aspartic acid and glutamic acid, two natural amino acids that have exactly the same functional groups and differ by glutamic acid being one methylene group longer than aspartic acid.
Anyway this paper caught my eye, both as a one time peptide chemist and as a person very interested in the sequestration of carbon in useful and environmentally more benign polymers.
From the abstract of the paper:
The engineering of biological molecules is a key concept in the design of highly functional, sophisticated soft materials. Biomolecules exhibit a wide range of functions and structures, including chemical recognition (of enzyme substrates or adhesive ligands1, for instance), exquisite nanostructures (composed of peptides2, proteins3 or nucleic acids4), and unusual mechanical properties (such as silk-like strength3, stiffness5, viscoelasticity6 and resiliency7). Here we combine the computational design of physical (noncovalent) interactions with pathway-dependent, hierarchical ‘click’ covalent assembly to produce hybrid synthetic peptide-based polymers. The nanometre-scale monomeric units of these polymers are homotetrameric, ?-helical bundles of low-molecular-weight peptides. These bundled monomers, or ‘bundlemers’, can be designed to provide complete control of the stability, size and spatial display of chemical functionalities. The protein-like structure of the bundle allows precise positioning of covalent linkages between the ends of distinct bundlemers, resulting in polymers with interesting and controllable physical characteristics, such as rigid rods, semiflexible or kinked chains, and thermally responsive hydrogel networks. Chain stiffness can be controlled by varying only the linkage. Furthermore, by controlling the amino acid sequence along the bundlemer periphery, we use specific amino acid side chains, including non-natural ‘click’ chemistry functionalities, to conjugate moieties into a desired pattern, enabling the creation of a wide variety of hybrid nanomaterials.
"Click Chemistry" is chemistry, generally organic chemistry, that involves chemical reactions that take place very fast and in quantitative or nearly quantitative yields under easily accessible conditions. "Click" reactions represent only a small subset of known chemical reactions, but they are very important.
From the papers introduction:
Our bundlemer-based polymer chains exhibit a variety of unique features. Unlike high-molecular-weight synthetic polymers, our chains use small (roughly 3 kDa), easily synthesized peptide sequences that fold into designed tetrameric 4-nanometre bundles. The subsequent covalent assembly of these bundles yields polymers with micrometre-scale contour lengths. The design of ?-helical homo-oligomers has a long history, with both empirical de novo8 and computational9 methods being used. Here, computationally designed homotetrameric bundles with D2 symmetry10 present two reactive groups at each end, owing to chemical functionalization of the amino termini of the constituent peptides (Fig. 1a). Distinct homotetrameric bundles with complementary reactive functional groups are chemically linked (or ‘clicked’ together) to produce bundlemer chains.
A graphic with some images of these polymers along with a cartoon describing an example of the "click chemistry" utilized in assembling these polymers:
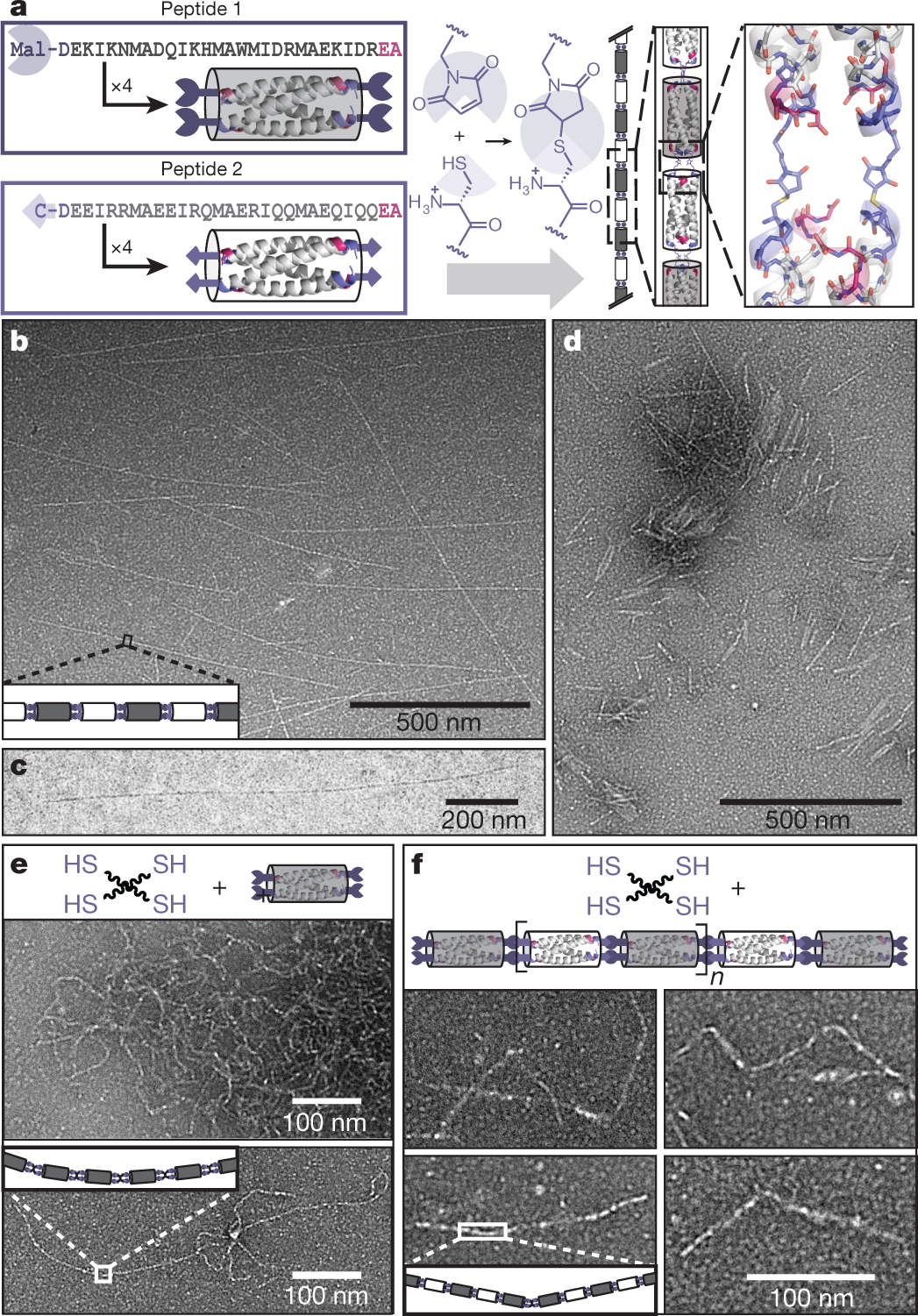
The caption:
a, Left, peptides 1 and 2 (Extended Data Fig. 1), shown in single-letter amino acid code, have at their N termini (blue) either maleimide (Mal) or cysteine (C). The carboxyl terminus (red) of each peptide is unreactive. Each sequence forms homotetrameric bundlemers: grey, peptide 1; white, peptide 2. Centre, the thiol–maleimide click reaction yields chains with two covalent linkages between neighbouring bundlemers. b, TEM of rigid rods produced with a 1/1 ratio of peptides 1 and 2. The sample is negatively stained with phosphotungstic acid (PTA). c, CryoTEM of rigid rods longer than 1 ?m in aqueous solution. d, Negatively stained TEM of short rigid-rod chains produced using an asymmetric ratio (10/9: [peptide 1]/[peptide 2]) of reacting bundlemers. e, The organic tetrathiol PETMP (black wavy lines) links peptide-1 bundlemers to form semiflexible chains. f, Examples of segmented chains produced by connecting short rigid rods with PETMP. Rod segments within the segmented polymers range in length from approximately 50 nm (where n, the number of bundlemers per segment, is approximately 3 to 4) to 100 nm (where n is approximately 8 to 9).
In this case the "click chemistry" involves the side chain of a very important amino acid, cysteine, which features a sulfhydryl side chain. (This amino acid is often involved in complexation of metals by metalloproteins, and is a frequently a critical feature of their catalytic sites. The affinity of mercury and cadmium for these thiols in lieu of the zinc with which they are supposed to function is a key factor in the toxicology of these two metals.)
The behavior of some of these polymers as liquid crystals:
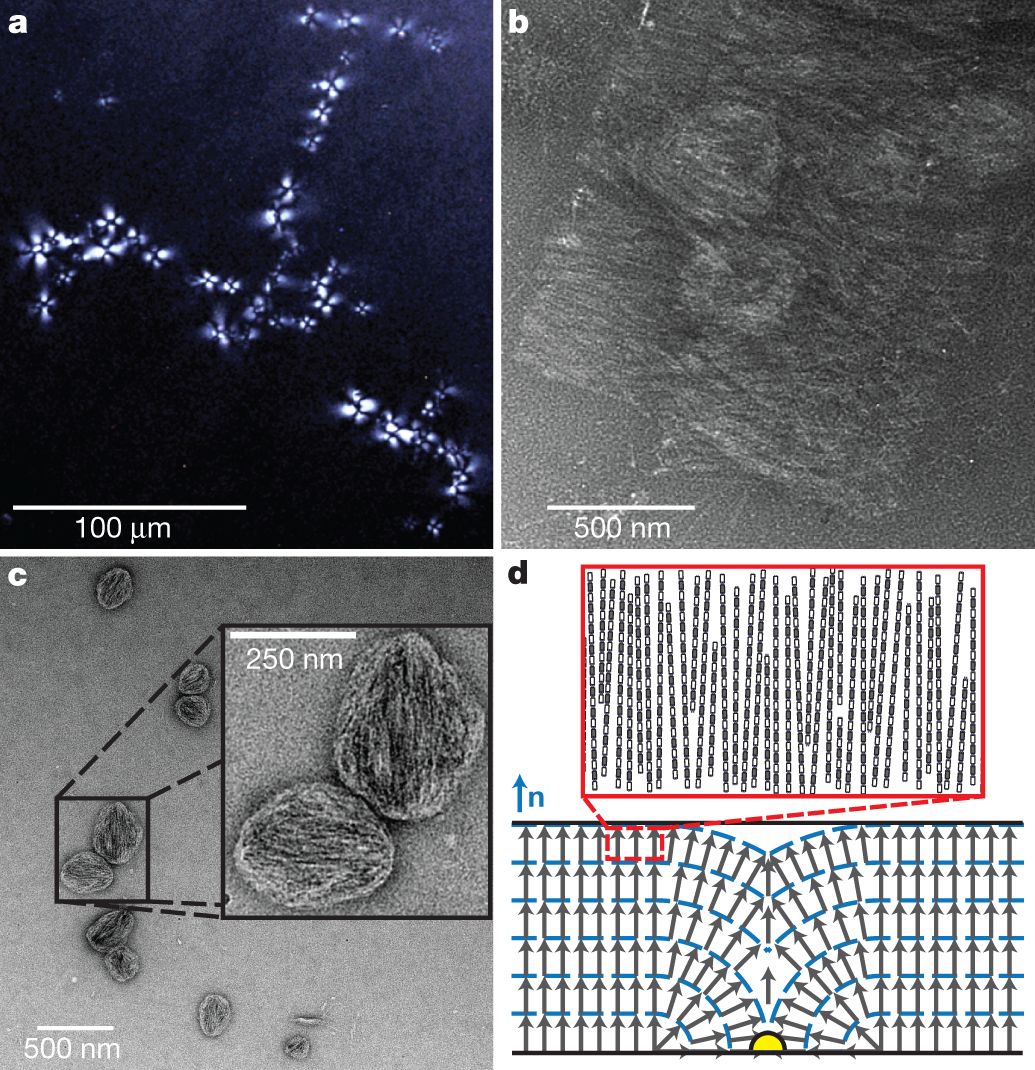
The caption:
Rods were prepared as in Fig. 1a, d with alternating bundlemers of peptide 1 and peptide 2. a, Polarized optical microscopy (POM) of a pseudoisotropic region of roughly 100-nm-long rods with multiple TFCDs, indicative of a lyotropic lamellar phase. POM was performed on an 8% (w/v) short rigid-rod solution at pH 2. b, TEM of negatively stained short rigid rods from the pseudoisotropic region shown in a, revealing clear rod layering. c, TEM reveals the structure of dilute regions in which rigid rods have locally aggregated into droplets with clear rod orientation. d, Bottom, diagram of a TFCD cross-section formed in smectic-A-type liquid crystals. Top, enlargement of a single smectic layer, showing the proposed homeotropic alignment of individual rigid rods. The blue dashed lines represent boundaries between smectic layers confined between parallel walls (thick black lines represent the glass slide and cover slip in the POM). The liquid-crystal director n, the axis along which all rods are aligned within individual layers, is perpendicular to the smectic layers. The local orientation director (grey arrows) within the smectic A layers is parallel to n far from the TFCD. In the vicinity of a topological defect on the glass substrate (yellow), the local orientation field folds towards the defect.
Some interesting reversible behavior of some of these polymers:
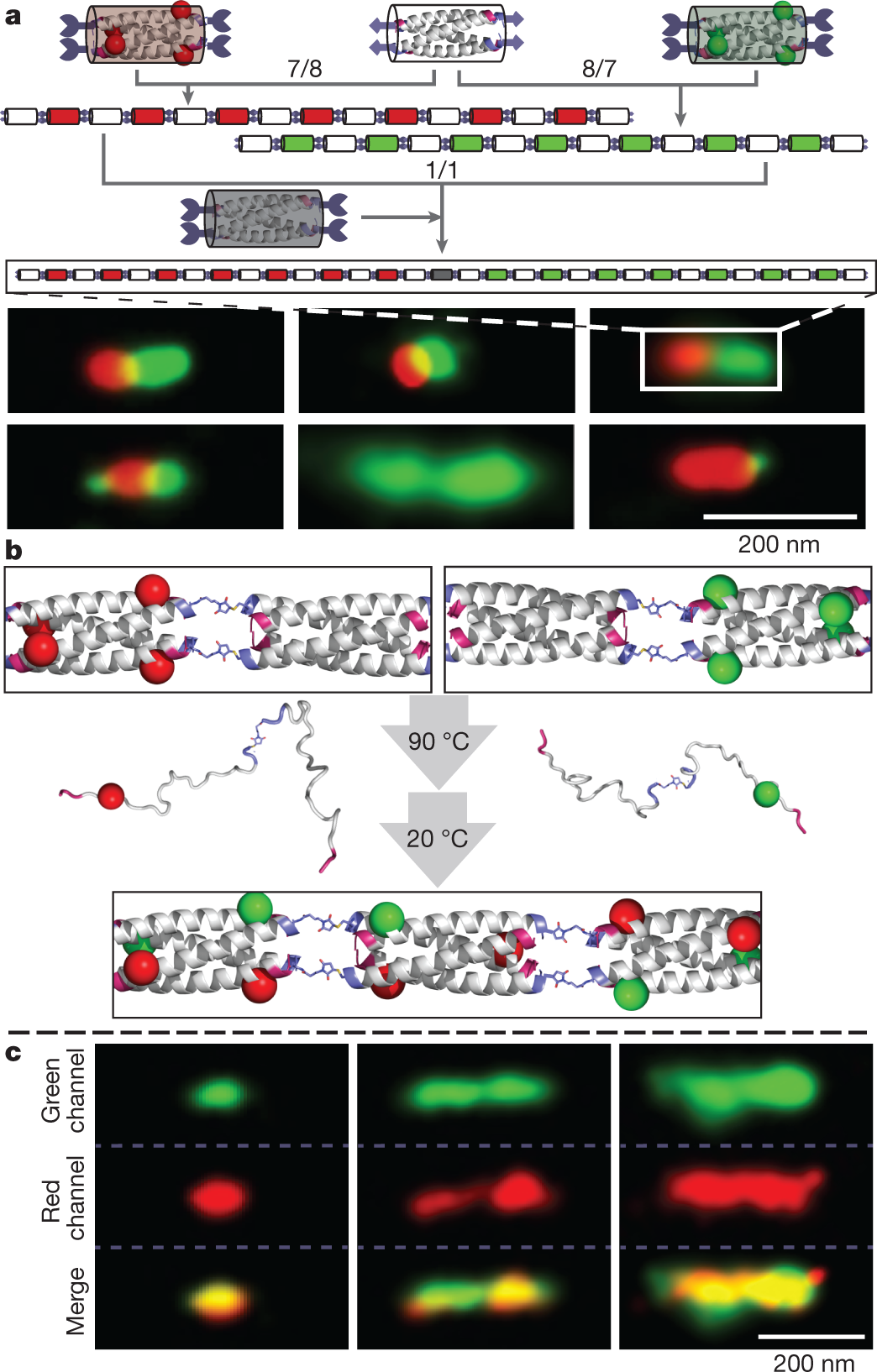
The caption:
a, Rigid rods were created using fluorescently labelled variants (peptide 3 (right) and peptide 4 (left); Extended Data Fig. 1), each containing either 4-chloro-7-nitrobenzofurazan (green) or 5(6)-carboxy-tetramethylrhodamine (red) attached to the lysine-24 side chain. Bundlemers of peptide 2 (centre, white) were used to form short rigid rods comprising a single dye type. The resulting red and green rods were joined with peptide-1 bundlemers (grey) to make longer rods with red and green segments. The STORM images below are of resulting longer rigid rods. The constituent red or green fluorescence of each segment is easily resolved. b, Rigid rods from a are heated to 90?°C, resulting in unfolding and dissociation of the individual bundles while peptide dimers remain covalently linked. When the solution is then cooled to 20?°C, the bundlemers and rigid rods reform. c, Reassembled rods now display co-localization of green and red fluorescence (producing a yellow signal when the green and red channels are displayed concurrently) along the entire reformed rod lengths.
Some other interesting properties suggesting hybrid material options:
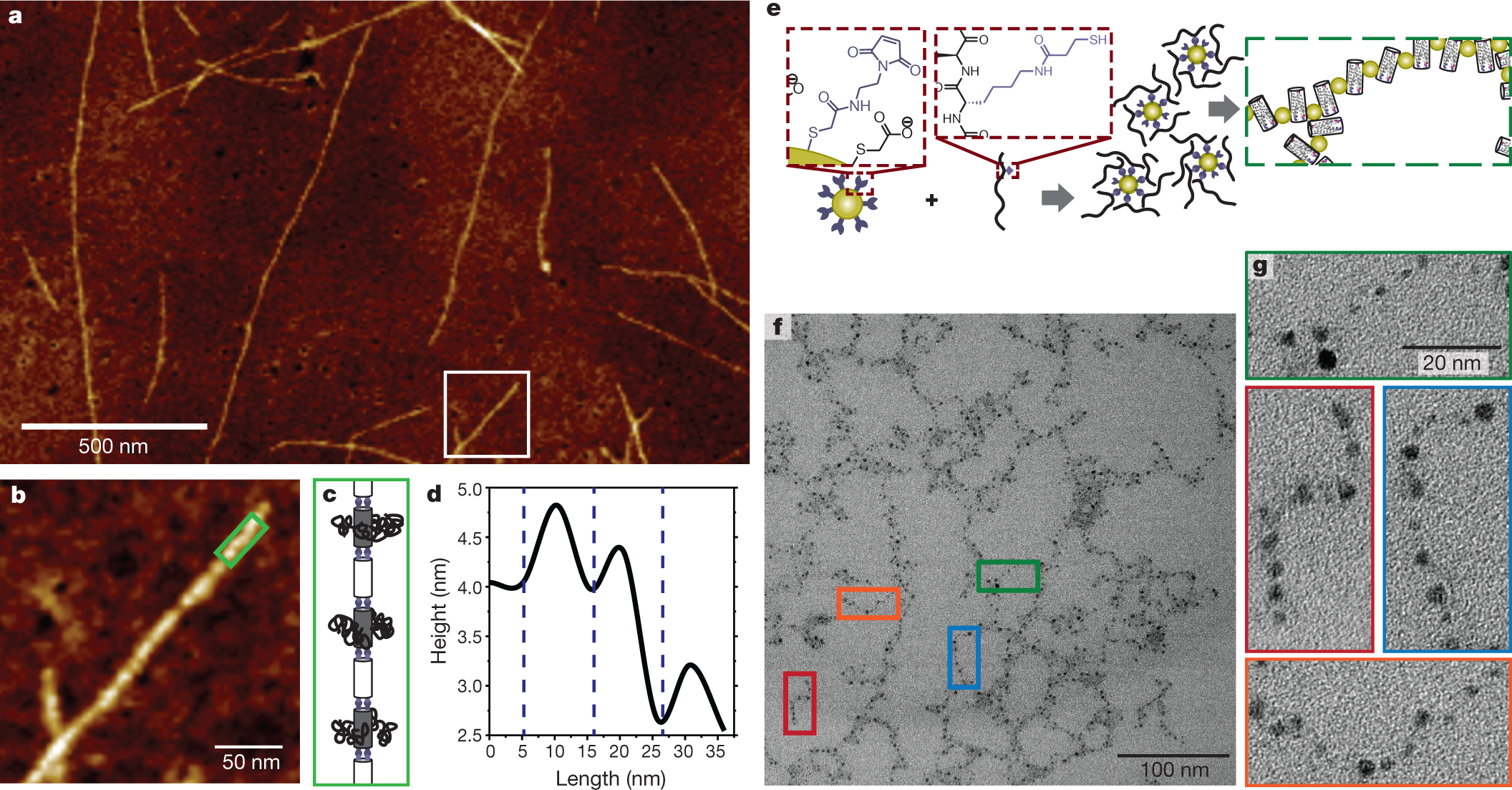
The caption:
a, AFM image of rigid rods formed using peptides 2 and 6 (Extended Data Fig. 1), with azide-functionalized PEG2000 chains conjugated to the rigid rods. b, AFM image of the rigid-rod area within the white outline in a; the area in the green rectangle was used for height analysis along the rod longitudinal axis (d). c, Diagram illustrating bundles of peptide 6 (grey) and peptide 2 (white) conjugated with PEG2000. d, Height trace along the longitudinal axis in b. e, Left, maleimide-functionalized gold nanoparticles are conjugated with peptide 7 (Extended Data Fig. 1), and then allowed to assemble into hybrid nanoparticle–bundlemer chains (right). f, TEM of nanoparticle–bundlemer chains. g, Magnified TEM images of the indicated nanoparticle–bundlemer chains in f reveal interparticle separation consistent with the dimensions of peptide bundles.
Here the co-ordinated nanoparticles are gold, but basically pretty much all of the metallic portions of the periodic table might prove accessible to such controlled polymers, offering the possibility of separations with very high distribution coefficients, useful for recovering dilute materials and also for remediating polluted sites.
Have a nice day tomorrow.



