Science
Related: About this forumA Study of the Extraction of Plutonium and Neptunium into Ionic Liquids.
The paper to which I'll refer is this one: Sequestration of Np(IV) and Pu(IV) with Hexa-n-octyl nitrilotriacetamide (HONTA) in an Ionic Liquid: Unusual Species vis-à-vis a Molecular Diluent Rajesh B. Gujar, Bholanath Mahanty, Seraj A. Ansari, Richard J. M. Egberink, Jurriaan Huskens, Willem Verboom, and Prasanta K. Mohapatra Industrial & Engineering Chemistry Research 2024 63 (1), 480-488.
Regrettably I won't have very much time to spend discussing this paper but will endeavor to make a few points about this critical task, chemical separations of the components of used nuclear fuel to extract all of the valuable components, not the least of which are the transuranium actinides, neptunium, plutonium and americium. The paper is about the first two of these. To my mind it is of critical importance to exploit all of the actinides that can be obtained from used nuclear fuel. In particular, neptunium, and to a lesser extent americium, have important properties that will allow for the irreversible denaturation of weapons grade plutonium in any successful nuclear weapons disarmament program.
Let me preface the excerpting by saying I am not really a solvent extraction kind of guy, although I am very much interested in the properties of nuclear materials, especially the actinides, in ionic liquids, which have wide electrochemical windows and are not subject to flammability and to vapor pressures. Still the paper is interesting, nice to see. I certainly agree emphatically with the first sentences in the opening paragraphs. From the introduction:
In the quest for clean energy with low carbon emissions, nuclear energy is considered to be one of the most viable options and its contribution to the world energy demand is going to grow at a much rapid rate. (1?6) Though nuclear energy appears quite attractive, its sustainability can be achieved with a closed fuel cycle, which involves reprocessing of the spent nuclear fuel. (7?10) Furthermore, the global acceptance of nuclear energy is dependent on the efficiency at which large volumes of highly radioactive wastes are processed. The reprocessing of spent nuclear fuel involves the “PUREX” (Plutonium Uranium Redox Extraction) process that uses tri-n-butyl phosphate (TBP) in a paraffinic diluent as the solvent. (11) Though TBP is the work horse of the nuclear industry for over half a century, there are several drawbacks, namely, significant aqueous solubility, poor radiolytic stability, deleterious nature of its degradation products, and generation of large volumes of solid wastes, which triggered the research on the development of alternative solvent systems that can be considered as “green”, i.e., with C, H, O, and N type extractants. (12,13) These efforts, started in the sixties of the last century, (14?16) have suggested that dialkyl amides are very good alternatives to TBP due to their complete incinerability and innocuous nature of their degradation products. Several researchers have evaluated many such dialkyl amides and concluded that these extractants are much more efficient than TBP under certain conditions. (17?19) Though plant-scale applications are lacking, there is a good possibility that the dialkyl amides can replace TBP in the near future at least for fuels containing a high Pu content. (20?22)
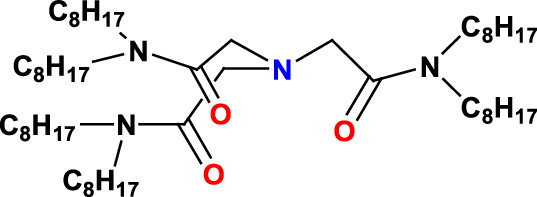
Cooperative complexation hypothesis suggests that multifunctional ligands can be far more efficient than ligands containing one ligating moiety. (23) Reports by Sasaki et al. have suggested that tripodal amide ligands such as N,N,N?,N?,N?,N?-hexa-n-octyl nitrilotriacetamide (HONTA) is one of the most efficient extractants for the extraction of actinide ions such as UO22+ and Pu4+ from nitric acid feeds. (24) The authors have also reported selective extraction of trivalent actinides vis-à-vis the trivalent lanthanides due to binding of the “soft” actinide ion to the soft donor “N” atom making the ligand tetradentate. (25,26) Other research laboratories have also studied the extraction behavior of HONTA and its homologs with varying alkyl chains with some very interesting results. (27?29) Though Np is not considered important in the PUREX process, as it escapes to the raffinate stream, its chemistry needs to be understood when amide-based extractants are used for spent fuel reprocessing. In this context, one needs to study the extraction behavior of Np, mainly in its +4 oxidation state with HONTA as well. To the best of our knowledge, a detailed understanding of this is not reported to date by any research group.
The studies involving HONTA, reported so far, utilized molecular diluents such as n-dodecane or its mixture with iso-decanol. In recent years, room temperature ionic liquids (termed as RTILs or simply ILs), a class of neoteric diluents, have been suggested as alternative “green” diluents due to properties like nonflammability, wide electrochemical window, higher radiolytic stability and benign nature. (30?34) RTILs are known to extract metal ions much more efficiently than the molecular diluents to the extent that in certain cases the D (distribution ratio) values are reported to be 3–4 orders larger than those obtained with molecular diluents. (35) There is no report available where HONTA has been used for the extraction of tetravalent actinide ions with RTIL-based diluents. This work deals with the use of both a molecular diluent and an ionic liquid for the extraction of the Np(IV) ion from an aqueous nitric acid medium. A comparison is done with the extraction behavior of Pu(IV) ion as well. Apart from the liquid–liquid extraction studies, cyclic voltammetric studies were also carried out to understand the redox behavior in the RTIL medium...
The structure of HONTA:
The ionic liquid used - there are many thousands of ionic liquids possible, along with the related deep eutectic solvents - is the well known and often discussed 1-butyl-3-methyl imidazolium bis(trifluoromethane sulfonyl)imide (Bumim·Tf2N). The paper discusses the electrochemical utility of this system by running cyclic voltammetry, a process that allows for the reduction and oxidation of metals - much as is done in batteries - to change their chemical properties. This particular work involves nitric acid solutions, and thus reduction of neptunium to the metal cannot be carried out, nor is their any reason to suspect the authors were interested in doing so.
The cyclic voltammogram and UV/VIs spectra:

The caption:
Personally I am very interested in the metallic forms of plutonium and neptunium, since they form together a low melting eutectic that suggests use of a very unusual fuel system in which I am trying to interest my son. (He says, "Dad, graduate students are unlikely to get to play with liquid plutonium! It's certainly not the focus of his lab groups, but before I die I want to leave this in the back of his mind.) I'm hoping that he'll realize his goal of working in a national laboratory and will weasel his way into getting to play with liquid plutonium and its alloys.
As a fuel, a metallic liquid plutonium/nepturnium alloy would allow for the nearly instantaneous and pretty much irreversible denaturation of weapons grade plutonium into a form of plutonium that is totally unacceptable for use in nuclear weapons.
Anyway, the authors find that the nature of the HONTA complexing agent is completely different in ionic liquids than it is in traditional molecular solvents, explored in this case with n-decane and isodecane. This is unsurprising.
Have a nice day tomorrow.