Science
Related: About this forum"Win a heart" science contest. Explain the difference between Faradaic efficiency and Thermodynamic efficiency.
This could be a short thread since it should be easy to get one or more of four DU hearts I have to give away. Alternatively it could be a short thread because no one is interested in it.
Unlike the rules set by the trivia person over in the Lounge, you are free to use Google to tell us what the difference between Faradaic efficiency and thermodynamic efficiency is in electrolysis. The first correct answer gets a heart from me. Upon the award of the heart, that part is closed with two parts to remain.
In scientific papers discussing approaches to making electrolysis to produce hydrogen economically viable - which it isn't - so we can all pretend in spite of reality that hydrogen is "green," - people often speak of "Faradaic Efficiency." (The numbers often seem impressive.) Faradaic efficiency is different however from "Thermodynamic efficiency," the latter being what antinuke Guru Amory Lovins told us, in 1976, would save the world, except it didn't. (He was apparently confused when he opened a physics text and assumed that no one else on the planet knew physics so he could claim he did.)
The consequence of thermodynamics has resulted in this industrial reality, to which I often point:
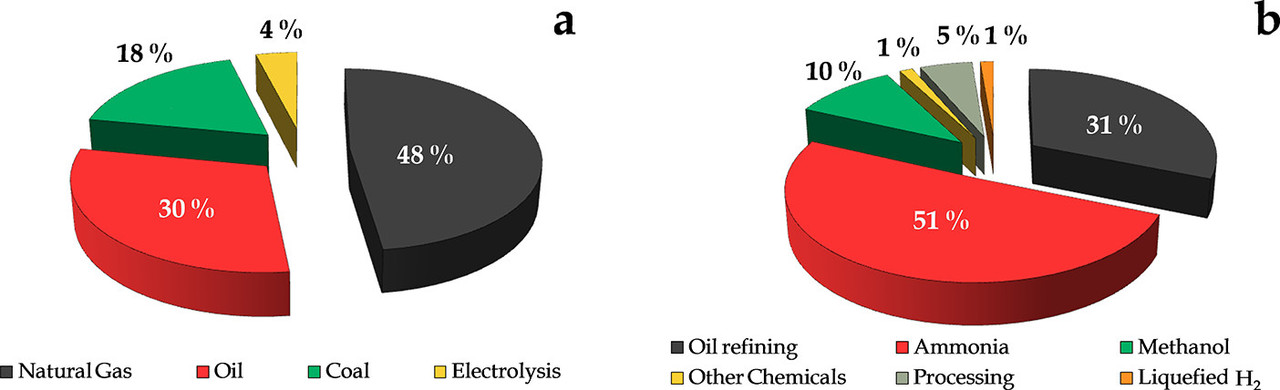
The caption:
Progress on Catalyst Development for the Steam Reforming of Biomass and Waste Plastics Pyrolysis Volatiles: A Review Laura Santamaria, Gartzen Lopez, Enara Fernandez, Maria Cortazar, Aitor Arregi, Martin Olazar, and Javier Bilbao, Energy & Fuels 2021 35 (21), 17051-17084]
You may also win a heart for producing here the equation that describes the thermodynamics of electrolysis.
You may win two hearts for showing, with a simple example, how this equation applies to the electrolysis of water to make hydrogen and oxygen, with brief satisfactory text on the implications, thus demonstrating why electrolysis, despite the efforts of fossil fuel salespeople and salesbots here and elsewhere to claim dishonestly otherwise, electrolysis is an economic nonstarter industrially and hydrogen production is dominated by fossil fuel dependence.
There will only be one award in each category.
Unfortunately, I am the judge of the contest as well as the person with hearts to give away, for what it's worth.
Lochloosa
(16,066 posts)NNadir
(33,525 posts)consider_this
(2,203 posts)Is that for unwanted hair removal, right?
haha, I know better in this context, but am suddenly in Dad joke mode.
I might read your challenge and try... later ![]()
NNadir
(33,525 posts)consider_this
(2,203 posts)Faraday efficiency (also called faradaic efficiency, faradaic yield, coulombic efficiency or current efficiency) describes the efficiency with which charge (electrons) is transferred in a system facilitating an electrochemical reaction.
Thermodynamic efficiency, is the maximum electrical energy available in the fuel, as calculated by the change in the Gibbs free energy divided by the change in enthalpy of the reaction.
got some definitions, now I can think about what you are saying and learn something - thank you!
Not vying for hearts, hoping to see if my brain still works - LOL
NNadir
(33,525 posts)consider_this
(2,203 posts)I fear I am too tired to try to figure this out. I do hope to learn what you are hoping to educate on here, so I am staying tuned to your thread - oh and a healthy kick!