Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Science
Related: About this forumRecovering Lanthanides (Rare Earths) From Acid Mine Drainage.
Recovery of Rare Earth Elements from Acid Mine Drainage with Supported Liquid Membranes: Impacts of Feedstock Composition for Extraction Performance Andrew Middleton, Benjamin C. Hedin, and Heileen Hsu-Kim Environmental Science & Technology 2024 58 (6), 2998-3006.Over the last several months, as papers on the subject pile up, happily at an increasing pace, on the subject of removing uranium from aqueous matrices, seawater and groundwater and to a lesser extent, riverine water, where it often occurs, in all three places, naturally as an example of "NORM" (naturally occurring radioactive materials), as well as from anthropogenic sources, uranium mine associated runoff, reprocessing sites generally devoted to weapons manufacture, and coal ash, in which uranium is often a prominent constituent, I have been working on a long post to discuss these many interesting approaches to the idea. Most of these are based on resins, often functionalized with amidoximes, but many other approaches are also discussed in the literature, extending even to proteins found in corals that are known to complex - for what evolutionary purpose I do not know - uranium. I may never get around to publishing it, but I often write posts at DU to force myself to look at the details, as opposed to superficial notation, of particular technologies devoted to a sustainable world, the world which, unfortunately, we do not live, but is, I believe, feasible to create, if unlikely given our mythology driven world culture.
I will not live to see a sustainable world, but hopefully I can hand off some of these ideas, if they have merit, to those who will follow and live with the environmental disaster now well underway.
I often write, in terms of energy, of "process intensification" wherein matrices at high temperatures provided by nuclear fuels are cooled in multiple processes that recover useful products and goals, clean desalinated water, clean portable fluid fuels (DME generally), landfill elimination, and downstream, as a side product, electricity. The idea is that if one can achieve multiple goals with one process or device, one should do so; this is the essence, to my mind, of sustainability.
One of the things we are leaving to the unfortunate generations who will follow us, is mine waste. We of course, consider this waste, but our future generations may have to pick through our garbage merely to survive, as we will have impoverished them. We generally think of mine waste in terms of tailings - some of which contain elements for which we have only recently found important industrial uses, indium for example in zinc mine tailings - but contaminated water is another product of mines.
As far as mines go, things will get much worse before they get better. It is a popular, albeit dangerous, reactionary and rather stupid idea, that so called "renewable energy" is sustainable. It isn't. The term "renewable energy" is essentially an oxymoron: The land intensity and the mass intensity driven by short lifetimes, low energy to mass ratios, and the need for redundancy to address the inherent lack of reliability of the weather - particularly in a period where we have dangerously destabilized the weather - make "renewable energy" not only lack sustainability, but is actively driving the acceleration of environmental degradation. So called "renewable energy" is nothing more than a highly questionable promotion on dependence on mining on land, and now we see, with Norway's recent disastrous decision, at sea.
I am thoroughly convinced of this, despite the unpopularity of my immutable opinion.
It is a huge mistake to confuse popularity with wisdom.
Among the many mined materials on which the so called "renewable energy" industry depends - it is hardly limited to so called "renewable energy" but extends to many other industries - are the lanthanides, in popular parlance, "the rare earth" elements. As is widely discussed in the critical materials issue in academic, industrial and government circles, the world overwhelmingly relies on Chinese mines for these elements, and there is a huge effort, worldwide, to source them elsewhere. The paper at the outset considers recovering them from a well known (and growing) source of water pollution, "acid mine drainage."
From the introductory text of the article:
Rare earth elements (REE) are critical materials for a large number of modern industries, (1) such as energy, defense, and electronic industries. (2,3) Further, their unique chemistry makes them currently irreplaceable in products such as high strength permanent magnets, hard-disk drives, petroleum cracking catalysts, location guidance systems, glass, and personal electronics, among others. (1?3) Due to unstable global supply markets and increasing demand, there is growing interest in recovering REE from waste residuals. (3?5) Proposed secondary resources include acid mine drainage (AMD), electronic wastes, and coal fly ash, all of which have been shown to be enriched in REEs. (6?9) The valorization of waste materials also has the added benefit of reducing the environmental burden associated with mining of primary ores. (10?13) Despite these benefits, these waste sources are low-grade in terms of REE purity and are often spatially distributed, factors that impose challenges for the selective extraction of REEs in an economically feasible manner. In this study, we evaluated the effectiveness of supported liquid membrane (SLM) separation as a method for selectively recovering REEs from AMD feedstocks.
AMD is generated from the dissolution and oxidation of sulfide minerals in waste rock piles and exposed rock formations produced during mining activities. Water that continuously leaches from these mined areas is enriched in soluble minerals and metals such as REEs, creating a potential source of critical metals. (8,9,14) In the northern Appalachia region of the United States alone, hundreds of abandoned coal mines collectively release 500–3400 t of REE annually. (9,15,16) While the total loading of REE into watersheds is large, the aqueous concentrations of REEs can be relatively low. AMD fluids with the highest concentrations of REE tend to have low pH (i.e., less than pH 4). (9) Even for these low-pH and REE-enriched AMD fluids, the aqueous composition is complex: total REE concentrations are exceeded by up to 5 orders of magnitude by major metals such as Fe, Al, Ca, and Mn. (8) As such, the development of separation processes for AMD and other low-grade feedstocks requires an understanding of the impact of these impurities and other water quality variables on REE recovery processes.
Separations by SLMs are a modification of solvent extraction, where the extraction and stripping processes have been combined into one unit operation. In SLM separations, the feedstock and product solutions are separated by a hydrophobic membrane that has been impregnated with an organic extraction solvent, such as di(2-ethylhexyl)phosphoric acid (DEHPA), dissolved in an oil phase...
AMD is generated from the dissolution and oxidation of sulfide minerals in waste rock piles and exposed rock formations produced during mining activities. Water that continuously leaches from these mined areas is enriched in soluble minerals and metals such as REEs, creating a potential source of critical metals. (8,9,14) In the northern Appalachia region of the United States alone, hundreds of abandoned coal mines collectively release 500–3400 t of REE annually. (9,15,16) While the total loading of REE into watersheds is large, the aqueous concentrations of REEs can be relatively low. AMD fluids with the highest concentrations of REE tend to have low pH (i.e., less than pH 4). (9) Even for these low-pH and REE-enriched AMD fluids, the aqueous composition is complex: total REE concentrations are exceeded by up to 5 orders of magnitude by major metals such as Fe, Al, Ca, and Mn. (8) As such, the development of separation processes for AMD and other low-grade feedstocks requires an understanding of the impact of these impurities and other water quality variables on REE recovery processes.
Separations by SLMs are a modification of solvent extraction, where the extraction and stripping processes have been combined into one unit operation. In SLM separations, the feedstock and product solutions are separated by a hydrophobic membrane that has been impregnated with an organic extraction solvent, such as di(2-ethylhexyl)phosphoric acid (DEHPA), dissolved in an oil phase...
Let's stop here. These "SLM" (Supported liquid membranes) are made by soaking a polyvinylidene fluoride, a polymer that is related to teflon, in kerosene containing a lanthanide complexing agent used in the chemical extraction of lanthanides, di(2-ethylhexyl)phosphoric acid (DEHPA). These are placed directly into samples of the contaminated water for the experiments, but not in the flowing mine drainage streams. This information may impact the environmental profile of this particular scheme for using a liquid membrane, but the reader should be aware that there are many other approaches besides "SLM" or even this particular variety of an "SLM;" the important issue is the quantity of elements that can be theoretically (or even practically) recovered from acid mine drainage, not the details.
The authors select, as their test sites shown here:

The caption:
Figure 1. Locations of AMD sample collection sites.
The metals and other species in the water at the seven abandoned mines are described in a table in the paper which follows:
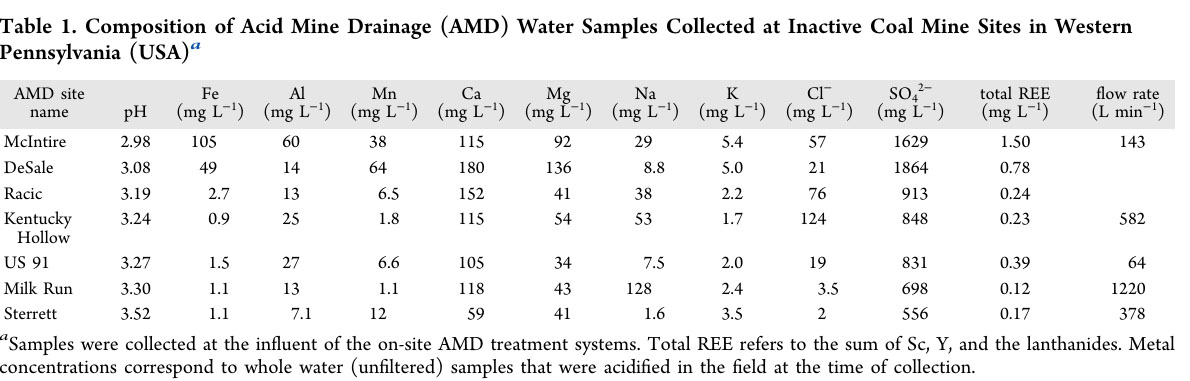
One should note that the amount of lanthanides and the two congeners that are found with them, scandium and yttrium, which are not "f elements" but rather "d elements" but are always found with the lanthanides, is dwarfed by the amount of iron, manganese, and in particular calcium and sodium.
I have loaded the data in this table into a spread sheet, and from the flow rates available for five of the seven mines - flow rates at the Desale and Racic mines are not reported - I have produced an estimation of how much of each of the cationic (metallic) elements flow in a year.
Approximately 3300 tons of iron flow out of the five mines in a year.
Approximately 8300 tons of aluminum flow out of the five mines in a year.
Approximately 2500 tons of manganese flow out of the five mines in a year.
Approximately 49,000 tons of calcium flow out of the five mines in a year.
Approximately 22,000 tons of magnesium flow out of the five mines in a year.
Approximately 39,000 tons of sodium flow out of the five mines in a year.
Approximately 1200 tons of potassium flow out of the five mines in a year.
The fourteen lanthanide elements plus yttrium and scandium total 221 tons out of the five mines in a year.
The supplementary information of the paper gives the breakdown of the 16 elements. Between 45% and 51% are represented by just two elements in the separate mine drainage, yttrium and cerium. Between 63% and 66% are represented by adding neodymium to these two, between 71% and 77% are covered by adding lanthanum to these three, and between 77% and 81% are covered by adding gadolinium.
All of these elements are useful and have markets, but they are not quite as critical as some of the others, in particular, dysprosium.
I have discussed dysprosium here:
Material Flow Analysis of Dysprosium in the United States
...and here:
Uncovering the Key Features of Dysprosium Flows and Stocks in China
If China is concerned about dysprosium availability, everyone should be concerned about it.
Overwhelmingly modern generators and electric motors utilize neodymium-iron-boride magnets, which heat up in use, accounting for some of the thermodynamic inefficiencies of generators and electric motors. Heat can degrade the quality of the magnetic fields of permanent magnets, and so, to stabilize the magnetic strength of iron, small amounts of dysprosium is added to them to stabilize their magnetism under high temperature conditions.
One hears of an "energy transition" frequently as if one exists. It doesn't exist; we are more dependent on dangerous fossil fuels than we have ever been. One hears that dysprosium is a critical element for the mythical "energy transition" and certainly the quixotic quest for so called "renewable energy" does raise demand for dysprosium in wind turbines, where it is stranded when the wind isn't blowing, but generators in dangerous coal powered plants, dangerous natural gas powered plants, and dangerous petroleum powered plants also use dysprosium laced magnets, as do the magnets in hair blowers, fans, electric cars, drink mixers, audio speakers, etc., etc., etc., on and on and on. One can, of course, make magnets from say, just iron, but the cost of doing so will be environmental, the energy efficiency of all of these devices will be lower and thus the environmental impact higher.
The five mines studies leach out about 600 kg, less than a ton, of dysprosium every year. The price of dysprosium in recent times has varied between roughly $400/kg and $750/kg in recent years, but one should note that this represents isolated dysprosium, separated from all of the other 15 elements in the lanthanide fraction including yttrium and scandium. In fact most of the economic value of the lanthanides lies with dysprosium, neodymium, and praseodymium.
Dysprosium demand is roughly about 8000 tons per year.
It is thus doubtful that the cost of recovery systems for these elements can be covered by their sale, but this may not be the main reason for accomplishing the recovery. The reason for removing the metals might be something that is often neglected in discussions of the embrace of technology which are generally only addressed in purely materialistic terms, cost. For instance, advocates of the failed and useless solar and wind industries often like to crow that they're "cheap" even though they require redundant systems which default to the use of dangerous fossil fuels, albeit coupled with soothsaying about vast mining enterprises in the future. (Mines depend on fossil fuels.) Thus the real cost, known as the external cost, the cost to the environment and human health is treated as if it doesn't exist, hence 7 million air pollution deaths a year and a planet in flames. Wind and solar are not "cheap" even if the electricity produced by them for short periods cannot be sold at a decent price, and in fact, no electricity can be sold at a decent and sustainable price, insuring that everybody loses.
Thus I would like to suggest that the recovery of elements from dilute sources like acid mine waste be undertaken for a different reason than satisfying bourgeois affectations: Decency. Future generations will be required to recover metals from dilute sources, waste heaps, and things like this mines. When we mutter obscenely about putative nirvanas (that never arrive) "by 2000" and then when 2000 comes, "by 2020" and then when 2020 comes "by 2040" and so on ad infinitum, what we are saying is that future generations should do what we have not bothered to do ourselves. Hey folks, rather than foist responsibility on our grandchildren, how about we take action ourselves. The side product of removing metals from streams of water is clean water.
This is the same as is the case with uranium. It is cheaper to mine uranium than to recover it from seawater, or from water supplies either naturally or as the result of anthropogenic isolation and processing. However if we remove uranium from natural resources, then we need to mine less or perhaps not mine any at all.
I do not expect an ethical world; at the end of my life, experience teaches me that expecting ethics to dominate decision making is a fool's errand, particularly in the age of the triumph of blank indifferent materialism. This said, an ethical world is feasible if not likely, and thus I like the focus of this paper.
Have a nice evening.
InfoView thread info, including edit history
TrashPut this thread in your Trash Can (My DU » Trash Can)
BookmarkAdd this thread to your Bookmarks (My DU » Bookmarks)
0 replies, 327 views
ShareGet links to this post and/or share on social media
AlertAlert this post for a rule violation
PowersThere are no powers you can use on this post
EditCannot edit other people's posts
ReplyReply to this post
EditCannot edit other people's posts
Rec (2)
ReplyReply to this post