Science
Related: About this forumFast Clean Separation of Americium and Europium.
The paper I'll discuss in this post is this one: First Report on a 1-Cycle Solid-Phase Extraction Method for Selective Separation of Am3+ and Eu3+: Use of BTP-Grafted Silica Decorated on Activated Carbon Seraj A. Ansari, Rajesh B. Gujar, Arunasis Bhattacharyya, Bharathkumar Thangaraj, Karthikeyan Natesan Sundaramurthy, Cingaram Ravichandran, Subba Rao Toleti, and Prasanta K. Mohapatra
Industrial & Engineering Chemistry Research 2024 63 (7), 3256-3264.
Europium, possibly because of its ready reduction to its divalent state, is one of the rarest and and most expensive of the lanthanides.
This point is made in another paper in the same issue of Industrial & Engineering Chemistry Research as the above mentioned paper appears, this one: Selective Separation of Europium from Lanthanum, Cerium, and Iron Using Porous and Functionalized Polymer, Maha A. Youssef, Emad H. Borai, Abeer El-khalafawy, and Mahmoud G. Hamed Industrial & Engineering Chemistry Research 2024 63 (7), 3152-3162
From that paper:
In fission products, isotopes with a mass number of 151 represent about 0.8% as a mole fraction, and in theory, non-radioactive isotopically 151Eu can be obtained by isolating radioactive 151Sm, and leaching it out as the 151Sm decays.
The paper sited at the outset discusses the separation of the main actinides in used nuclear fuel using the historical Purex process, a solvent extraction process that I personally think should be replaced with fluoride volatility approaches. Both processes will leave behind both europium and americium, f-element congeners, as a residue. Since all europium isotopes are strong absorbers of neutrons, in order to utilize americium as a fuel to exploit some rather spectacular approaches to weapons nonproliferation, the congeners must be separated.
The method in the paper cited at the outset of this post strikes me as pretty cool, since it's more or less flow chemistry. From the introductory paragraphs of that paper:
ARTICLE SECTIONSJump To
Nuclear power is rapidly emerging as the most reliable alternative power source to conventional sources because of its low carbon emission burden on the environment and sustainability due to the in situ production of fissile materials, viz., 239Pu. (1?3) One of the major challenges faced in the nuclear industry is the safe handling of the highly radioactive mass known as fission and the activation products. (4) Recycling of the unspent fissile 235U by the closed fuel cycle along with the thus generated 239Pu is one of the most accepted strategies for the sustainable growth of nuclear power. (5,6) The widely used technology for spent fuel reprocessing is the PUREX (Plutonium Uranium Redox EXtraction) process, (7) which involves the selective extraction of U and Pu from the fission products as well as the activation products consisting of the minor actinides, viz., Np, Am, and Cm. The PUREX raffinate is concentrated and acid-killed, resulting in a high-level liquid waste (HLLW) whose management has attracted the attention of scientists and technologists working on the back-end processes. While the present practice of the HLLW management involves conversion of the waste oxide to vitrified glass blocks, which can be kept under surveillance after burial in deep geological repositories, R&D efforts have indicated eased surveillance period by a fast-evolving strategy termed as “actinide partitioning” followed by the transmutation of the long-lived minor actinides. (8?11) However, the extractions of trivalent minor actinides have serious interference from the trivalent lanthanides, requiring an effective method for the separation of the trivalent f-cations.
The separation of trivalent actinides and lanthanides is one of the most difficult tasks in separation chemistry due to their similar chemical properties. (12?16) Although much progress has been made concerning the development of extractants, the liquid–liquid extraction process has certain drawbacks, such as excess utilization of volatile organic compound (VOC)-based solvents, secondary waste accumulation, emulsion formation between phases, and multistage extraction processes, which will affect the economic performance of the process in large-scale practical applications. Alternatively, solid-phase extraction technique (17) has been considered as a relatively “green” separation method with high separation efficiency due to the possibility of column mode operation and easy scale-up options. Extraction chromatography, which uses solid adsorbents whose pores contain the selective organic extractant for selective extraction of the metal ion of interest, has attracted the attention of researchers working in the area of nuclear fuel cycle processes due to simplicity, ease of operation, recovery of elements, stability and reusability of absorbent, safety with respect to hazardous reagents/samples, and consumption of less organic solvents, which have a positive impact on the economics and safety toward extraction of spent nuclear fuel radionuclides. (18)
A large number of reports on solid absorbents using alumina, zeolites, polymers, impregnated resins, silica, magnetic nanomaterials, and carbon-based materials (graphene oxide, CNT, and activated carbon) have appeared in the literature. (19?21) Activated carbon (AC) is a popular choice of absorbent among all those commonly used owing to its high surface area, porous structure, nontoxic nature, low cost, adsorption capacity, surface reactivity, thermal as well as chemical stability, and unlimited availability of natural carbon sources (agricultural waste). (21?23) Conceivably, the chemical modification of AC materials is an adaptable method to enhance their selectivity toward specific analytes. (24?26) Most frequently, –OH, –CHO, –COOH, and –COOR groups are utilized in the modification procedures.
In our previous studies, we synthesized a few hydrophilic BTP ligands (SO3PhBTP, SO3PhBTBP, and SO3PhBTPhen) and investigated their efficacy for Am3+ and Eu3+ separation via solvent extraction as well as supported liquid-membrane (SLM) studies. (12) Encouraged by these results, and in order to evaluate the scope and limitations of the solid-supported BTP, a novel BTP-embedded activated carbon AC–Si–BTP was prepared for the first time. In this approach, R–BTP was synthesized and anchored onto an AC surface using silica (Si–R) as a linker. The surface aryl groups of AC have been exploited, and surface-anchored BTP can contribute to extraction; hence, a potential enhancement in minor actinide separation is expected. As represented in Figure 1, the aim of the present work is to selectively load Am3+ onto the AC–Si–BTP composite material making it a direct 1-cycle separation process with the BTP-based compound in the same way Cyanex 301-based solid-phase extraction materials reported in the literature. (27) The only BTP-based solid-phase extraction method for Ln–An separation was extraction chromatography using a tripodal diglycolamide (T-DGA), which used an aqueous soluble BTP (SO3PhBTP) as the complexing eluent, making it a 2-cycle separation process. (28)...
A crude schematic of the preparation of the solid phase extraction matrix:
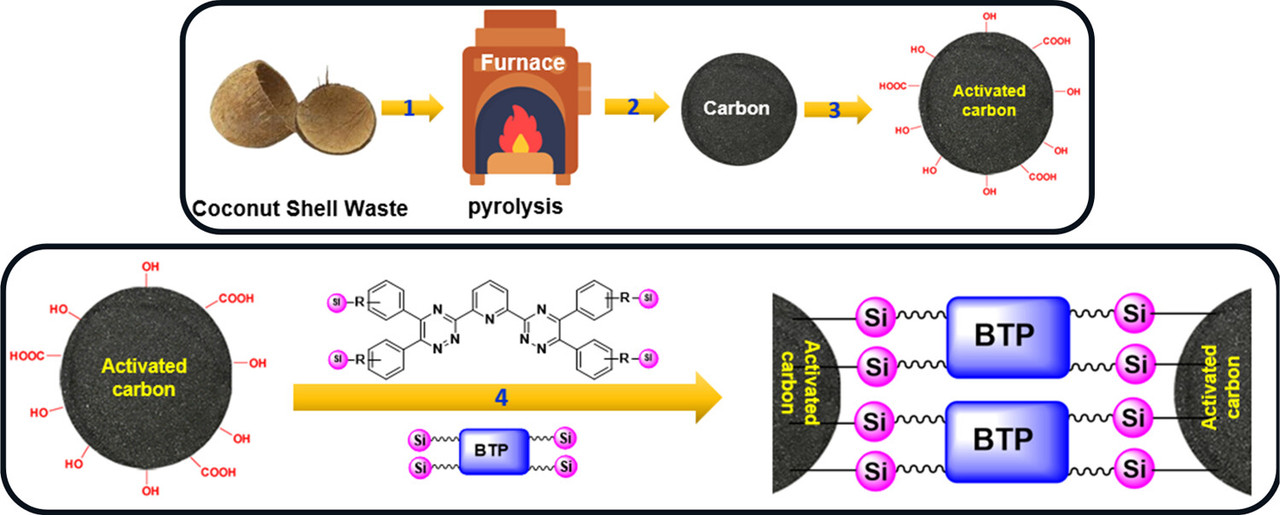
The caption:
The separation factors as a function of pH of the eluents.
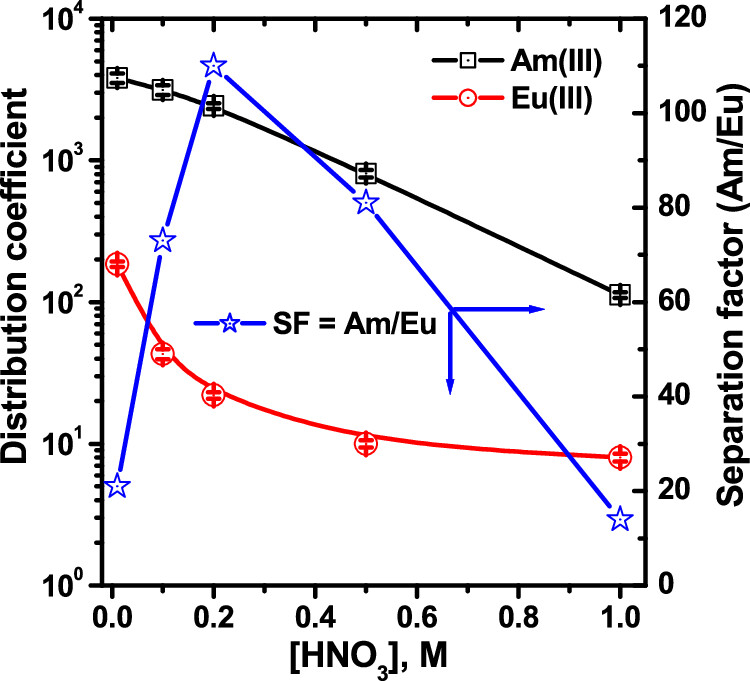
The caption:
Depending on the mechanical system used, the solid phase exhibits good performance over multiple cycles.
Although over the long term, for various reasons, mainly connected with isotope separation of radioactive and non-radioactive species, for instance the isotopes of palladium, I favor the rapid recycling of hot fuels, initially we will want to learn about these processes using relatively old fuels, of which we have a great deal as a unexpectedly happy circumstance. Because Am arises from the long term decay of 241Pu, older fuels will have more americium than younger fuels, and europium's long lived radioactive isotope 152Eu, will have also largely decayed to stable gadolinium.
I have previously discussed the critical masses of the major americium isotopes elsewhere:
Critical Masses of the Three Accessible Americium Isotopes.
Regrettably I won't have time to go deeper into this paper, nor will I have time to discuss the wonderful properties of americium as a nuclear fuel.
Have a nice day tomorrow.